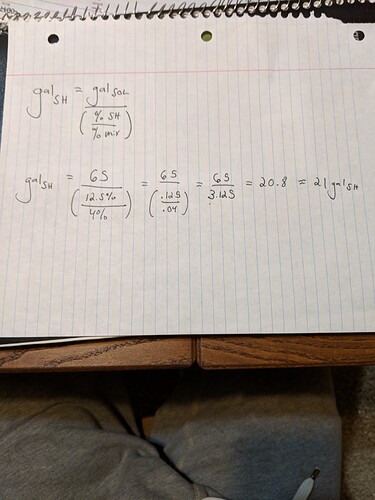I forgot to put a 0 there. Its 90/10. Per 100 gallons. This is for direct application.
Just keep reading and the answers you seek you shall find.
… I would’ve been kicked out ages ago ![]()
This is a classic. I love it.
You’re grandfathered in lol
Simple Dilution formula:
C1xV1=C2xV2
Where C is concentration (in this case %SH)
And V is volume (gallons in this example)
So to get from 12.5% SH down to 1% and we’ll say for a 5 gallon total solution:
12.5xV1=1x5
Algebra (divide both sides of the equation by 12.5)
(1x5)/12.5=V1=.4
So you find that you need to use .4 gallons or 51.2 oz (128 oz/gallon) and dilute up to 5 gallons total volume to achieve a 1% solution of SH
This method is easy and gives a CLOSE approximation. However it doesn’t take specific gravity (effectively excess NaOH in solution die to manufacturing process) into account so it will yield a slightly higher concentration solution than desired.
A more accurate way of calculating SH dilution requires you to measure the specific gravity of your concentrated SH. You can do this by weighing an accurately measured volume of SH and dividing the weight by the weight of that volume of water (8.345 lbs/gallon).
Using : Hx((A-B)/B)=X
Where H is the specific gravity
A is the initial SH concentration
B is target SH concentration
And X is the amount of water that needs to be added to each gallon of concentrated SH
So the same example as above but now using a reasonable specific gravity of 1.154
1.154x((12.5-1)/1)=13.271
So that means for every gallon of SH you need to add 13.271 gallons of water to go from 12.5% SH to 1% SH. Or a ratio of 13.271:1 water:SH
Now if we reduce that down to the 5 gallon total ratio used in the simple dilution example we get:
5/14.271=.35 so 13.271x.35=4.65
So 4.65 : .35 water:SH
This shows that when using the specific gravity I the dilution equation we actually only needed .35 gallons of 12.5% SH in a 5 gallon mix of 1% SH instead of the .4 gallons the simple dilution formula yielded. In my opinion this variance in concentration from the desired concentration is relatively negligible if you are doing your soft washing properly and pre-wetting plants and not over spraying.
Hope this helps. Sorry if the formatting is bad, this was my first post and done on a cell phone. I believe this information to be accurate but if someone thinks my math is incorrect please let me/every one know.
Had to replace * with x to indicate multiplication because some of the asterisks weren’t showing up in the post
Too complicated. 
That sounds really neat. A much more effective and simple method is to simply add more bleach if it isn’t coming clean
Well that’s fine and all but the request was for the formula…
Lol what “formula” are you talking about? Do you think there’s some special method for diluting something from 12.5% to 1%? It’s basic math. Or chemistry. Whichever way you want to look at it. It’s not like Avogadro sat down and developed some special formula that only works for diluting bleach when using it in a pressure washer.
Well, a stupid question definitely got an appropriate answer from you lol
There’s a 128 ounces in a gallon, so a gallon of 12.5% SH would have 16 ounces of actual SH to a gallon. If I want a 5 gallon pail of 3%, I would need 19 (approx) ounces of SH to 621 oz of water. Thus I would put 1.2 gallons of 12.5% into 5 gallon pail and fill the rest with water.
Sorry didn’t realize you had already provided a scientific answer I thought you were still asking for one. I made this out which works and I think is much easier to grasp.
And the knowledge about specific gravity is great, but like IBS said, just add more bleach. We’re not doing an experiment where quantities have to be so exact that you need to account for that.
Great info. Thank you! The whole idea behind my original request was to mix my own SH from pool shock in powder form. I can buy a 40lb. bucket of powder pool shock with a 73% SH concentration for less than $100 at my local pool supplier. Imagine how much 12% liquid SH I can make if I can figure this out. Way cheaper and way more convenient than buying gallon jugs. Or even filling my 75gal tank from my bulk supplier. So converting 73% concentrated SH in powder form down to 1 gallon of 12% liquid SH is the magic question. I believe you can purchase a kit that may do this and I think it uses similar formulas to yours above. All in all, great info in this thread. Thanks!
Do a search on the forum for either calcium chloride or powered SH before you go buying anything. It has been done before with terrible results.
Wow. Again way too much math for power washing. Just buy or make a proportioner and start spraying. Much easier to use the 100 scale like I previously posted. 10 gallons of bleach to 90 gallons of water. That will give you approximately a 1.25% ratio.
Also do not use calcium hypochlorite. You will have nothing but trouble. Find liquid sh.
Now back to camping…
Oxi-Clean is Calcium Chloride, or Calcium hypochlorite correct?
Please just stop with this.
What???
Are planning on using oxi clean? Wtf… liquid pool chlorine. That is what is used and needed. Stop over thinking this and making something that is easy into something that is so complicated. Calcium hypochlorite will leave a white residue over everything. Good luck if you want to go that route.