So I have done some additional research and would like @CaCO3Girl to validate if she could.
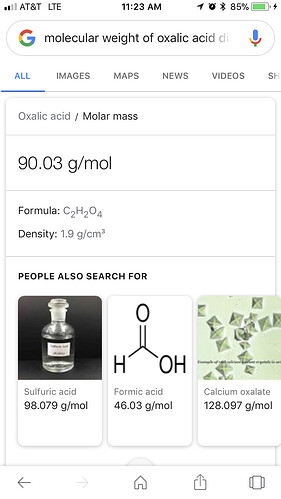
What we use is Oxalic Acid Dihydrate (powdered form). The molecular weight of the active acid is .714/gram.
To build a recipe to calculate a concentration strength % I used the following formula:
(Total weight in grams of OA * molecular weight)/volume weight (ml converted to gram) * 100 to get %.
Recipe
7.5 grams Oxalic Acid Dihydrate - this happens to be 1 tablespoon (not packed or mounded, just dip it in the bag and level it off).
100 ml water (happens to be 1/3 cup)
Formula -
(7.5*.714)/102/100 = 5.25%
This recipe will yield a 5.25% strength OA solution.
If this logic and understanding is true, then applying this to the real world would give us the following results -
The most common suggestion through anecdotal evidence in comments from forum post is to use 1/2 cup OA per gallon of water. Applying the formula we get the following:
1/2 Cup OA = 60 grams
1 gal water = 1280 ml
(60*.714)/1285*100 = 3.33%.
The results are that when it is suggested to use 1/2 cup OA per gallon of water, it relates to a 3.33% solution strength.
When a poster makes the comment that they used 1 cup and it was “HOT”, they were using a 6.66% solution. Note - this is neither good or bad, just relating what “HOT” means in terms of %. It is still no where near the maximum solubility of 14 % that @CaCO3Girl referenced above. At 14% would be have the equivalent of rocket fuel then? (Metaphorically not literally people)
Off Topic a bit - 1/4 up of OA powder is fatal to humans as it will shut down the liver terminally.
@CaCO3Girl = based on the 1/2 cup OA/Gal = 3.33% recipe and referring back to your comments of a “touch of phosphate and surfactant”, how much of each would you recommend to add to maximize efficiency and effectiveness of the solution in a production environment?
My next step in this learning process is to complete the following experiment:
Using a single piece of aged wood, cleaning using only light pressure and then apply various strengths of OA Solutions ( 1.65%, 3.33%, 4.99% and 6.66% - these % representing recipes using 1 gal of water and 1/4, 1/2, 3/4 and 1 cup OA powder) and applying to sections on the board to provide a results based effect of specific strengths using a constant basis (same board, same dwell times, same rinse times, etc) to determine best strength to use and/or effects of various strengths. The second part of this process, will be the let the board dry and then reapply the solutions to see what effect a second application has.
More to come on the same bat channel, same bat time!